Explore the connection between hydrochloric acid (HCl) and pH levels, from understanding pH basics to applications and safety precautions.
Understanding Hydrochloric Acid pH and Its Importance
Hydrochloric acid typically has a pH level that is highly acidic, usually ranging from around 0 to 1 on the pH scale. This extreme acidity is due to the high concentration of hydrogen ions present in the solution. However, the exact level of Hydrochloric Acid ph depending on its concentration and temperature. It’s important to handle hydrochloric acid with care due to its corrosive nature and to use appropriate safety precautions when working with it.
Definition of pH
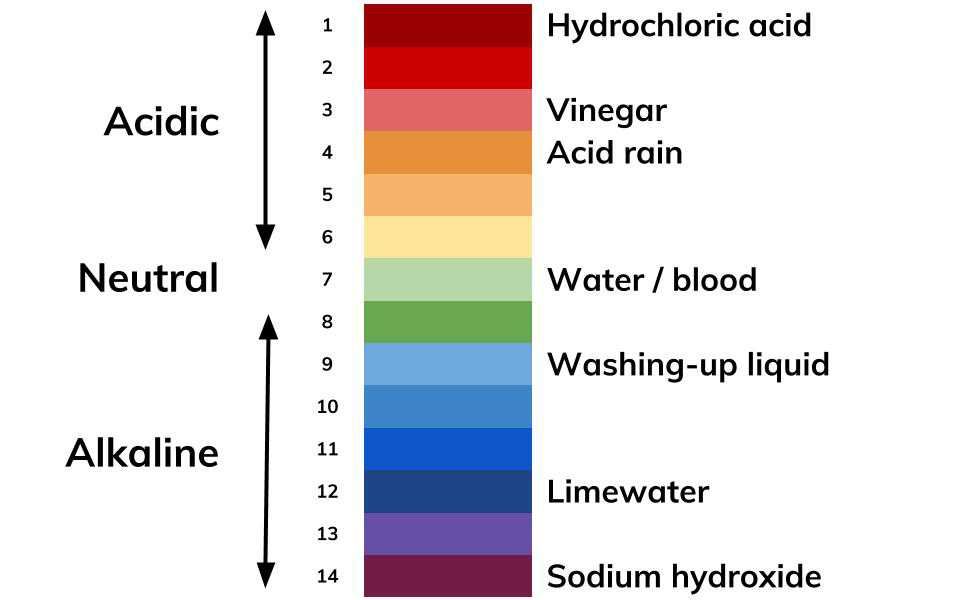
pH is a measure of the acidity or alkalinity of a solution, determined by the concentration of hydrogen ions (H⁺) present. The pH scale ranges from 0 to 14, with 7 being neutral, lower values indicating acidity, and higher values indicating alkalinity.
pH Scale
The pH scale is logarithmic, meaning each unit change represents a tenfold difference in acidity or alkalinity. For example, a solution with a pH of 3 is ten times more acidic than a solution with a pH of 4.
Role of pH in Chemistry and Biology
pH plays a crucial role in various chemical and biological processes, including enzyme activity, nutrient absorption, and cellular function. Maintaining proper pH levels is essential for overall health and biochemical balance.
pH Regulation in the Body
Acid-Base Balance
The human body tightly regulates pH levels to maintain homeostasis and ensure optimal physiological functioning. This balance is maintained through buffering systems, respiration, and renal function.
Physiological Functions
Optimal pH levels are essential for enzymatic activity, protein structure, and cellular metabolism. Deviations from normal pH ranges can disrupt biological processes and lead to health complications.
Hydrochloric Acid and pH
Acidic Nature of Hydrochloric Acid
Hydrochloric acid is a strong mineral acid with a low pH, typically ranging from 0 to 1. It dissociates in water to release hydrogen ions, contributing to its acidic properties.
Effect on pH Levels
Adding hydrochloric acid to a solution will decrease its pH, making it more acidic. This property makes hydrochloric acid valuable for pH adjustment and regulation in various applications.
Applications of Hydrochloric Acid in pH Regulation
Industrial Processes
Hydrochloric acid is used in various industrial processes, including metal cleaning, pickling, and chemical manufacturing, where precise pH control is essential for product quality and process efficiency.
Water Treatment
In water treatment plants, hydrochloric acid is utilized for pH adjustment and disinfection to ensure safe drinking water and prevent corrosion in distribution systems.
Laboratory Applications
In laboratory settings, hydrochloric acid is employed for pH adjustment, titrations, and chemical synthesis, providing accurate and reproducible results in analytical procedures.
Safety Precautions When Handling Hydrochloric Acid
Protective Gear
When handling hydrochloric acid, it is essential to wear appropriate personal protective equipment (PPE), including safety goggles, gloves, and a chemical-resistant apron, to minimize exposure and prevent injuries.
Storage and Handling
Hydrochloric acid should be stored in a well-ventilated area away from incompatible substances, such as metals, bases, and organic materials, in tightly sealed containers to prevent leaks and spills.
Disposal
Proper disposal of hydrochloric acid is crucial to prevent environmental contamination and hazards. Dispose of unused or diluted acid according to local regulations and guidelines.
Alternatives to Hydrochloric Acid for pH Regulation
While hydrochloric acid is effective for pH adjustment, there are alternatives available, such as sulfuric acid, phosphoric acid, and citric acid, which offer similar pH regulation properties with varying degrees of acidity and safety profiles.
Conclusion
Hydrochloric acid plays a significant role in pH regulation, with applications ranging from industrial processes to water treatment and laboratory experiments. Understanding the relationship between hydrochloric acid and pH levels is essential for safe handling and effective use in various applications. By following safety precautions and exploring alternative options, you can harness the power of hydrochloric acid for pH regulation while minimizing risks to health and the environment. When it comes to acquiring consumable laboratory supplies, including hydrochloric acid, it’s crucial to prioritize safety, quality, and regulatory compliance.